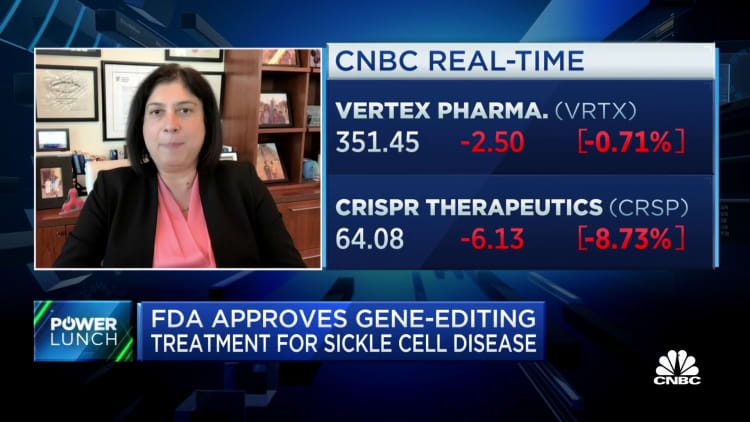
watch
now
The
U.S.
Food
and
Drug
Administration
on
Friday
approved
the
country’s
first
gene-editing
treatment,
Casgevy,
for
use
in
patients
with
sickle
cell
disease.
The
approval
comes
about
a
decade
after
the
discovery
of
CRISPR
technology
for
editing
human
DNA,
representing
a
significant
scientific
advancement.
Yet
reaching
the
tens
of
thousands
of
people
who
could
benefit
from
the
treatment
could
be
challenging
given
the
potential
hurdles
—
including
cost,
at
$2.2
million
per
patient
—
of
administering
the
complex
therapy.
Casgevy,
co-developed
by
Vertex
Pharmaceuticals
and
CRISPR
Therapeutics,
uses
Nobel
Prize-winning
technology
CRISPR
to
edit
a
person’s
genes
to
treat
disease.
The
treatment
was
approved
by
U.K.
regulators
last
month.
Shares
of
Vertex
fell
1%
Friday,
while
shares
of
CRISPR
fell
8%.
Sickle
cell,
an
inherited
blood
disorder,
causes
red
blood
cells
to
become
misshapen
half
moons
that
get
stuck
inside
blood
vessels,
restricting
blood
flow
and
causing
what
are
known
as
pain
crises.
About
100,000
Americans
are
estimated
to
have
the
disease.
This
microscope
photo
provided
on
Oct.
25,
2023,
by
the
Centers
for
Disease
Control
and
Prevention
shows
crescent-shaped
red
blood
cells
from
a
sickle
cell
disease
patient
in
1972.
Britain’s
medicines
regulator
has
authorized
the
world’s
first
gene
therapy
treatment
for
sickle
cell
disease,
in
a
move
that
could
offer
relief
to
thousands
of
people
with
the
crippling
disease
in
the
U.K.
Dr.
F.
Gilbert/CDC
via
AP,
File
Casgevy
uses
CRISPR
to
make
an
edit
to
a
person’s
DNA
that
turns
on
fetal
hemoglobin,
a
protein
that
normally
shuts
off
shortly
after
birth,
to
help
red
blood
cells
keep
their
healthy
full-moon
shape.
In
clinical
trials,
Casgevy
eliminated
pain
crises
in
most
patients.
The
FDA
approved
the
treatment
for
people
12
years
and
older.
“Sickle
cell
disease
is
a
rare,
debilitating
and
life-threatening
blood
disorder
with
significant
unmet
need,
and
we
are
excited
to
advance
the
field
especially
for
individuals
whose
lives
have
been
severely
disrupted
by
the
disease,”
said
Dr.
Nicole
Verdun,
director
of
the
Office
of
Therapeutic
Products
within
the
FDA’s
Center
for
Biologics
Evaluation
and
Research,
in
a
statement.
“Gene
therapy
holds
the
promise
of
delivering
more
targeted
and
effective
treatments,
especially
for
individuals
with
rare
diseases
where
the
current
treatment
options
are
limited,”
Verdun
added.
While
the
treatment
itself
is
administered
only
once,
the
whole
process
takes
months.
Blood
stem
cells
are
extracted
and
isolated
before
being
sent
to
Vertex’s
lab,
where
they’re
genetically
modified.
Once
ready,
patients
receive
chemotherapy
for
a
few
days
to
clear
out
the
old
cells
and
make
room
for
the
new
ones.
After
the
new
cells
are
infused,
recipients
spend
weeks
in
the
hospital
recovering.
Vertex
will
take
the
lead
on
launching
the
drug
and
estimates
about
16,000
people
with
severe
cases
of
sickle
cell
will
be
eligible.
Even
among
the
people
who
could
benefit
the
most,
analysts
worry
few
will
clamor
for
a
treatment
that
takes
months
to
complete,
carries
the
risk
of
infertility
and
could
be
cost
prohibitive.
Vertex
said
in
a
regulatory
filing
Friday
it
will
charge
$2.2
million
per
patient
for
the
treatment.
“We
believe
the
price
of
medicine
to
reflect
the
value
that
it
brings,
and
the
value
that
this
brings
is
a
one-time
therapy
for
potentially
a
lifetime
of
cure,”
Vertex
CEO
Dr.
Reshma
Kewalramani
said
Friday
in
an
interview
with
CNBC.
Vertex
is
seeing
“unanimous
enthusiasm”
from
payers,
patients
and
physicians,
because
people
with
sickle
cell
have
been
marginalized,
Kewalramani
said,
and
the
field
hasn’t
seen
much
innovation.
Because
the
procedure
is
so
complex,
it
will
be
limited
to
certain
health
facilities
like
academic
medical
centers.
Nine
health-care
facilities
are
ready
to
start
administering
Casgevy,
Vertex
said
in
a
release,
with
more
facilities
added
in
the
coming
weeks.
Bluebird’s
Lyfgenia
The
FDA
also
on
Friday
approved
a
separate
gene
therapy
by
Bluebird
Bio,
called
Lyfgenia that
works
differently
than
Casgevy
but
is
administered
similarly
and
is
also
intended
to
eliminate
pain
crises.
That
therapy
was
similarly
approved
for
the
treatment
of
sickle
cell
disease
in
people
12
years
and
older.
Bluebird
will
charge
$3.1
million
per
patient
for
Lyfgenia.
Shares
of
that
company,
which
has
a
market
value
of
just
about
$300
million,
fell
40%
Friday.
Dr.
Peter
Marks,
director
of
the
FDA’s
Center
for
Biologics
Evaluation
and
Research,
estimated
during
a
call
with
reporters
Friday
that
across
the
two
therapies
approved
Friday,
close
to
20,000
patients
will
be
eligible
for
treatment.
But
the
FDA
included
a
black-box
warning
–
the
strongest
safety
warning
label
–
to
Bluebird
Bio’s
Lyfgenia,
noting
that
in
rare
cases
the
therapy
can
cause
certain
blood
cancers.
The
FDA
added
that
warning
after
two
patients
who
received
Lyfgenia
in
a
clinical
trial
died
from
a
form
of
leukemia,
Verdun
told
reporters
Friday.
The
agency
said
it’s
still
unclear
whether
Lyfgenia
itself
or
another
part
of
the
treatment
process,
such
as
the
chemotherapy,
caused
the
cancer.
But
Marks
said
that
the
FDA
wants
patients
to
be
aware
of
all
potential
side
effects
of
the
entire
treatment
process:
“It’s
about
the
totality
of
the
therapy
that’s
given,”
he
told
reporters.
Vertex
did
not
see
similar
blood
cancer
cases
in
its
clinical
trial,
which
is
why
it
did
not
receive
a
black-box
warning
on
its
label,
Verdun
noted.
Both
Bluebird
Bio
and
Vertex
will
follow
patients
who
receive
the
treatments
for
15
years
as
part
of
a
post-approval
study.
The
FDA
has
encouraged
the
companies
to
specifically
monitor
for
malignancies,
or
the
presence
of
cancerous
cells
that
can
spread
to
other
sites
of
the
body.
